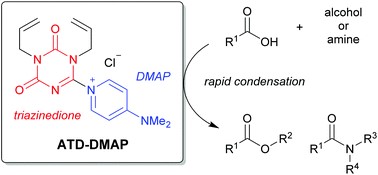
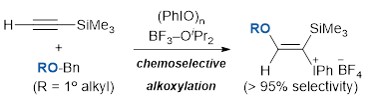
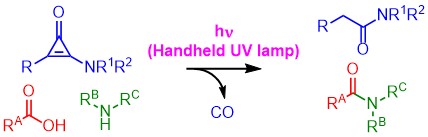
A versatile iodo(III)etherification of terminal ethynylsilanes using BF3-OiPr2 and alkyl benzyl ethers
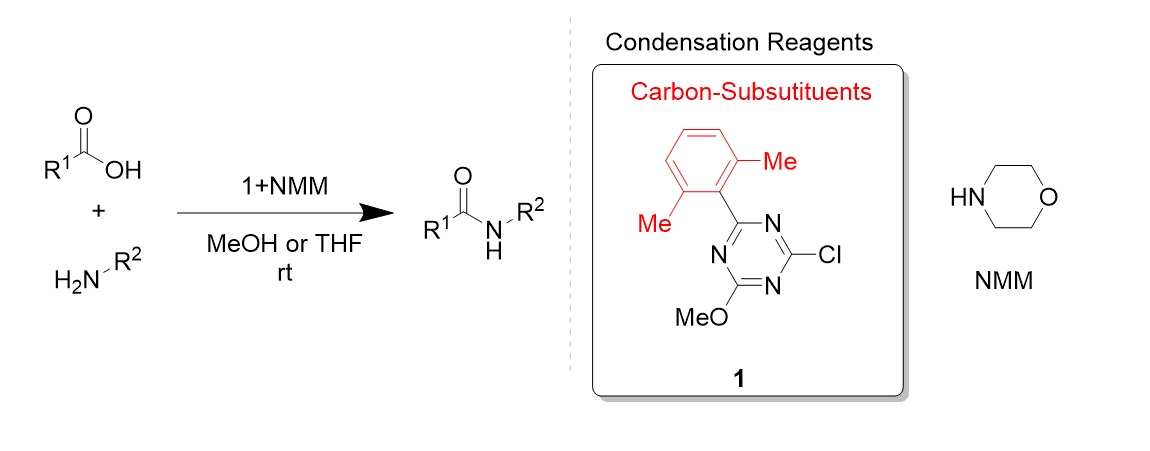
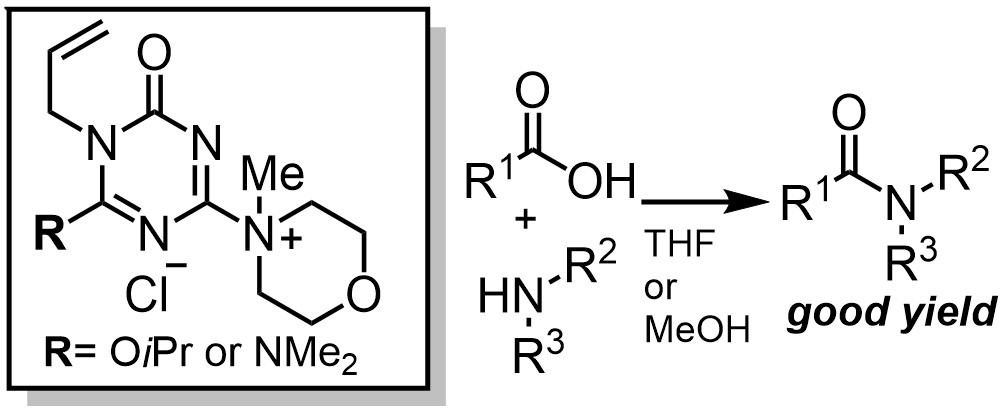
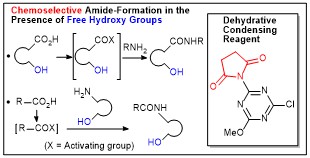
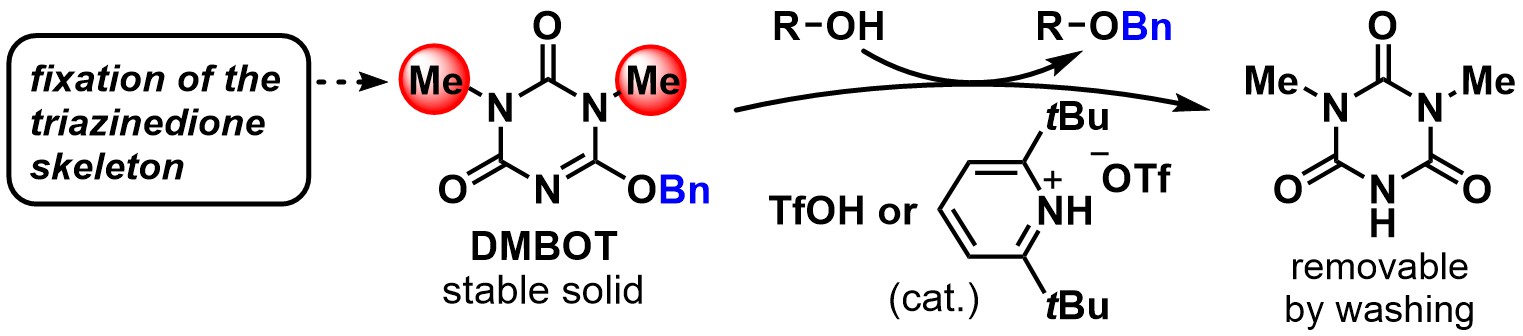
Development of hydrophilic polyacrylamide gel-based condensing reagents comprised of chlorotriazine
Kohei Yamada, Shota Hirozawa, Junqing Xia, Munetaka Kunishima
Chemical and Pharmaceutical Bulletin 68 (6) pp.534-537 (DOI:10.1248/cpb.c20-00074)
Chemical and Pharmaceutical Bulletin 68 (6) pp.534-537 (DOI:10.1248/cpb.c20-00074)
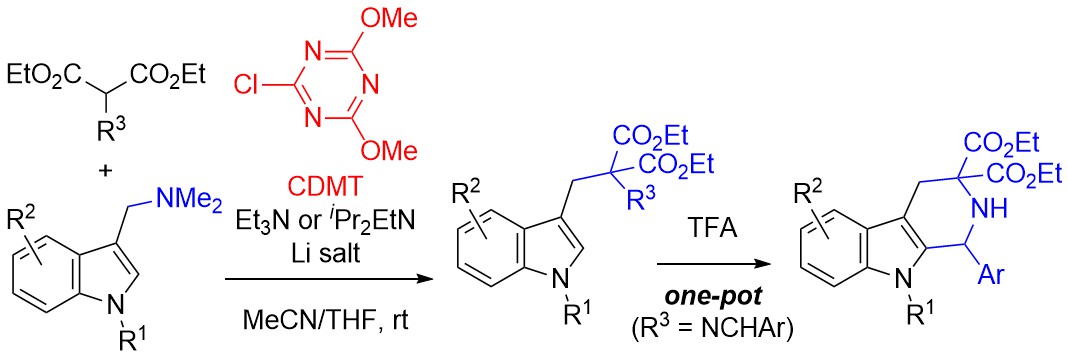
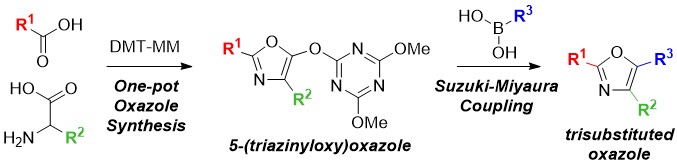
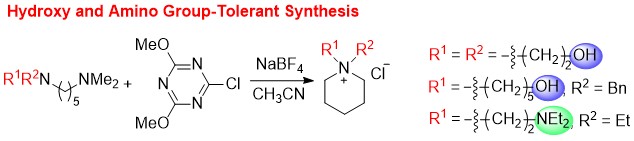
Substitution of the Dimethylamino Group in Gramines and One-Pot Cyclization to Tetrahydro-β-carbolines Using a Triazine-Based Activating Agent
Development of radioiodine-labeled acetaminophen for specific, high-contrast imaging of malignant melanoma
Wen Jing Zhu, Masato Kobayashi, Kohei Yamada, Kodai Nishi, Kotaro Takahashi, Asuka Mizutani, Ryuichi Nishii, Leo G Flores II, Naoto Shikano, Munetaka Kunishima, Keiichi Kawai
Nuclear Medicine and Biology 59 pp. 16-21 (DOI: 10.1016/j.nucmedbio.2017.12.008)
Nuclear Medicine and Biology 59 pp. 16-21 (DOI: 10.1016/j.nucmedbio.2017.12.008)
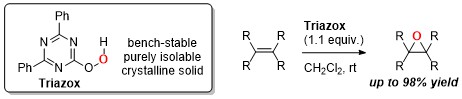
An isolable and bench-stable epoxidizing reagent based on triazine: Triazox
Kohei Yamada, Yuki Igarashi, Tatsuki Betsuyaku, Masanori Kitamura, Koki Hirata, Kazuhito Hioki, Munetaka Kunishima
Organic Letters 20 (7) pp. 2015-2019 (DOI: 10.1021/acs.orglett.8b00560)
化学ポータルサイトChem-Stationで紹介されました。
Organic Letters 20 (7) pp. 2015-2019 (DOI: 10.1021/acs.orglett.8b00560)
化学ポータルサイトChem-Stationで紹介されました。
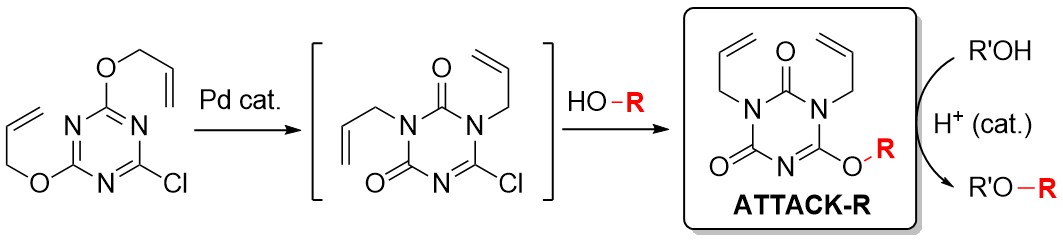
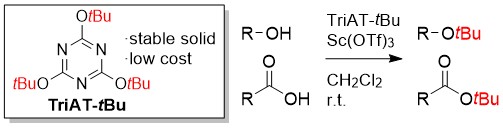
Development of Triazine-Based Benzylating Reagents Possessing t-Butyl Group on the Triazine Core: Thermally Controllable Reagents for the Initiation of Reaction
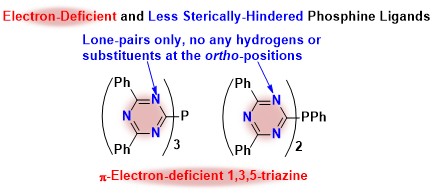
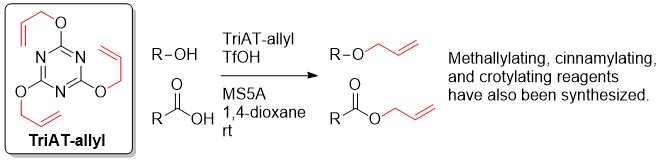
Novel Alkylating Reagents Designed by the Characteristics of 1,3,5-Triazines
Munetaka Kunishima, Kohei Yamada, Hikaru Fujita, Masanori Kitamura
Journal of Synthetic Organic Chemistry, Japan
Journal of Synthetic Organic Chemistry, Japan
75 (10) pp.1023-1034 (DOI: 10.5059/yukigoseikyokaishi.75.1023)
Increased Plasma Concentrations of Unbound SN-38, the Active Metabolite of Irinotecan, in Cancer Patients with Severe Renal Failure
Ken-ichi Fujita, Yusuke Masuo, Hidenori Okumura, Yusuke Watanabe, Hiromichi Suzuki, Yu Sunakawa, Ken Shimada, Kaori Kawara, Yuko Akiyama, Masanori Kitamura, Munetaka Kunishima, Yasutsuna Sasaki, Yukio Kato
Pharmaceutical Research 33 (2), pp. 269-282 (DOI: 10.1007/s11095-015-1785-0)
Pharmaceutical Research 33 (2), pp. 269-282 (DOI: 10.1007/s11095-015-1785-0)
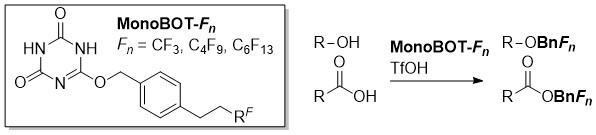
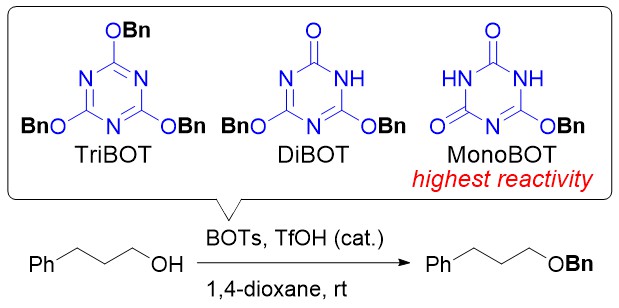
O-Benzylation of Carboxylic Acids Using 2,4,6-Tris(benzyloxy)-1,3,5-triazine (TriBOT) under Acidic or Thermal Conditions
Kohei Yamada, Saki Yoshida, Hikaru Fujita, Masanori Kitamura, and Munetaka Kunishima
European Journal of Organic Chemistry (36), pp. 7997-8002 (DOI: 10.1002/ejoc.201501172)
European Journal of Organic Chemistry (36), pp. 7997-8002 (DOI: 10.1002/ejoc.201501172)
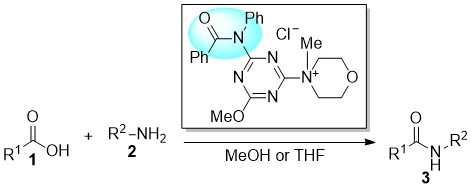
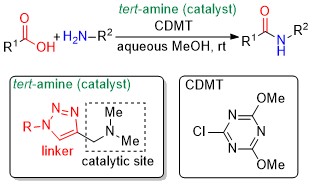
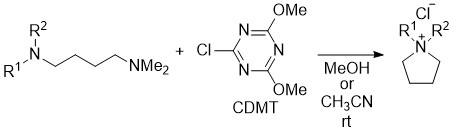
Role of Linkers in Tertiary Amines that Mediate or Catalyze 1,3,5-Triazine-based Amide-forming Reactions
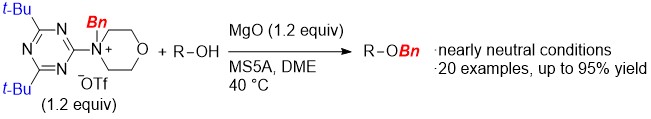
Development of a New Benzylating Reagent Spontaneously Releasing Benzyl Cation Equivalents at Room Temperature
Kohei Yamada, Yuichi Tsukada, Yukiko Karuo, Masanori Kitamura, and Munetaka Kunishima
Chemistry - A European Journal 20 (38), pp. 12274-12278 (DOI: 10.1002/chem.201403158)
Chemistry - A European Journal 20 (38), pp. 12274-12278 (DOI: 10.1002/chem.201403158)
Specific Labeling of Streptavidin for Better Understanding of Ligand Modification in Modular Method for Affinity Labeling(MoAL)
Munetaka Kunishima, Daiki Kato, Shuichi Nakanishi, Masanori Kitamura, Kohei Yamada, Keiji Terao, and Tomoya Asano
Chemical and Pharmaceutical Bulletin 62 (11), pp. 1146-1150 (DOI: 10.1248/cpb.c14-00468)
Chemical and Pharmaceutical Bulletin 62 (11), pp. 1146-1150 (DOI: 10.1248/cpb.c14-00468)
A Practical Method for p-Methoxybenzylation of Hydroxy Groups Using 2,4,6-Tris(p-methoxybenzyloxy)-1,3,5-triazine (TriBOT-PM)
Kohei Yamada, Hikaru Fujita, Masanori Kitamura, Munetaka Kunishima
Synthesis 45 (21), pp. 2989-2997 (DOI: 10.1055/s-0033-1339713)
Synthesis 45 (21), pp. 2989-2997 (DOI: 10.1055/s-0033-1339713)
Study of 1,3,5-Triazine-Based Catalytic Amide-Forming Reactions: Effect of Solvents and Basicity of Reactants
Munetaka Kunishima, Masanori Kitamura, Hiroyuki Tanaka, Ichiro Nakakura, Takahiro Moriya, Kazuhito Hioki
Chemical and Pharmaceutical Bulletin 61 (8), pp. 882-886 (DOI: 10.1248/cpb.c13-00368)
Chemical and Pharmaceutical Bulletin 61 (8), pp. 882-886 (DOI: 10.1248/cpb.c13-00368)
A New Method Using 2-Chloro-4,6-dimethoxy-1,3,5-triazine for Facile Elimination of Dimethylamino Group in Eschenmoser’s Methylenation for Synthesis of α,β-Unsaturated Esters
Kohei Yamada, Kazumasa Masaki, Yuri Hagimoto, Seina Kamiya, Munetaka Kunishima
Tetrahedron Letters 54, pp. 1758-1760 (DOI: 10.1016/j.tetlet.2013.01.092)
Tetrahedron Letters 54, pp. 1758-1760 (DOI: 10.1016/j.tetlet.2013.01.092)
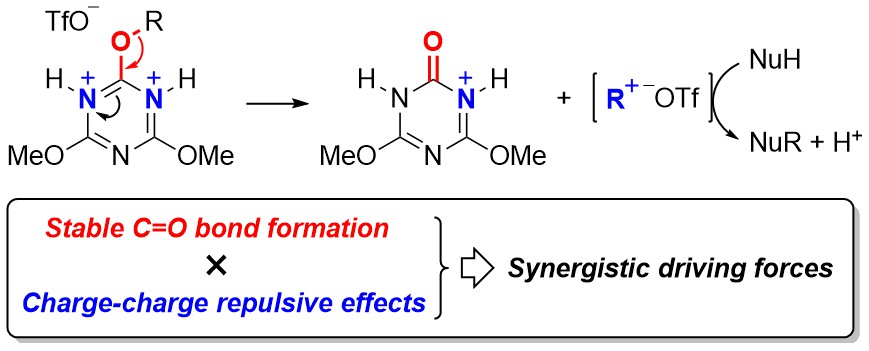
Study on 1,3,5-Triazine Chemistry in Dehydrocondensation: Gauche Effect on the Generation of Active Triazinylammonium Species
Munetaka Kunishima, Takae Ujigawa, Yoshie Nagaoka, Chiho Kawachi, Kazuhito Hioki, Motoo Shiro
Chemistry - A European Journal 18, pp. 15856-15867 (DOI: 10.1002/chem.201202236.)
Chemistry - A European Journal 18, pp. 15856-15867 (DOI: 10.1002/chem.201202236.)
A novel acid-catalyzed O-benzylating reagent with the smallest unit of imidate structure
Kohei Yamada, Hikaru Fujita, Munetaka Kunishima
Organic Letters 14 (19), pp. 5026-5029 (DOI: 10.1021/ol302222p)
Organic Letters 14 (19), pp. 5026-5029 (DOI: 10.1021/ol302222p)
N-isopropyl-p-iodoamphetamine hydrochloride is predominantly metabolized by CYP2C19
Ken-ichi Fujita, Minako Sugiyama, Yuko Akiyama, Kazuhito Hioki, Munetaka Kunishima, Kodai Nishi, Masato Kobayashi, Keiichi Kawai and Yasutsuna Sasaki
Drug Metabolism and Disposition 40 (5), pp. 843-846 (DOI: 10.1124/dmd.111.043893)
Drug Metabolism and Disposition 40 (5), pp. 843-846 (DOI: 10.1124/dmd.111.043893)
Substrate-selective dehydrocondensation at the interface of micelles and emulsions of common surfactants
Kunishima, M., Kikuchi, K., Kawai, Y., Hioki, K.
Angewandte Chemie International Edition 51 (9), pp. 2080-2083 (DOI: 10.1002/anie.201107706)
Angewandte Chemie International Edition 51 (9), pp. 2080-2083 (DOI: 10.1002/anie.201107706)
New approach to oligotriazoles using a cobalt complex of propargyl azides as a synthetic component
Yuichi Tsukada, Kohei Yamada, Munetaka Kunishima
Tetrahedron Letters 52 (26), pp. 3358-3360 (DOI: 10.1016/j.tetlet.2011.04.082)
Tetrahedron Letters 52 (26), pp. 3358-3360 (DOI: 10.1016/j.tetlet.2011.04.082)
Labeling study of avidin by modular method for affinity labeling (MoAL)
Shuichi Nakanishi, Hiroyuki Tanaka, Kazuhito Hioki, Kohei Yamada, Munetaka Kunishima
Bioorganic and Medicinal Chemistry Letters 20 (23), pp. 7050-7053 (DOI: 10.1016/j.bmcl.2010.09.109)
Bioorganic and Medicinal Chemistry Letters 20 (23), pp. 7050-7053 (DOI: 10.1016/j.bmcl.2010.09.109)
Effects of stereochemistry and β-substituents on the rates of vinylic SN2 reaction of hypervalent vinyl(phenyl)-λ3-iodanes with tetrabutylammonium halides
Kazunori Miyamoto, Takuji Okubo, Masaya Hirobe, Munetaka Kunishima, Masahito Ochiai
Tetrahedron 66 (31), pp. 5819-5826 (DOI: 10.1016/j.tet.2010.04.041)
Tetrahedron 66 (31), pp. 5819-5826 (DOI: 10.1016/j.tet.2010.04.041)
Convenient modular method for affinity labeling (MoAL method) based on a catalytic amidation
Munetaka Kunishima, Shuichi Nakanishi, Jin Nishida, Hiroyuki Tanaka, Daiki Morisaki, Kazuhito Hioki, Hiroshi Nomoto
Chemical Communications (37), pp. 5597-5599 (DOI: 10.1039/B912908A)
Chemical Communications (37), pp. 5597-5599 (DOI: 10.1039/B912908A)
Synthesis of aza-bridged calix(4-methoxy)triazines toward flattened π-conjugated macrocycles
Hiroyuki Tanaka, Ayako Wada, Motoo Shiro, Kazuhito Hioki, Daiki Morisaki, and Munetaka Kunishima
Heterocycles 79 (C), pp. 609-616 (DOI: 10.3987/COM-08-S(D)24)
Heterocycles 79 (C), pp. 609-616 (DOI: 10.3987/COM-08-S(D)24)
A Simple Practical Method for the Synthesis of 4,6-Dimethoxy-1,3,5-triazin-2(1H)-one Using Dimethylamine-Functionalized Solid-Phase Reagents
Kazuhito Hioki, Keiichi Ohshima, Yuko Sota, Miki Tanaka, Munetaka Kunishima
Synthesis (4), pp. 542-544 (DOI: 10.1055/s-0028-1083330)
Synthesis (4), pp. 542-544 (DOI: 10.1055/s-0028-1083330)